UPDATE 4/13/21: The Texas Department of State Health Services is asking vaccine providers in Texas to pause all administration of the Johnson & Johnson/Janssen COVID-19 vaccine following the CDC’s recommendation as it investigates six reported U.S. cases of a rare blood clot. For more information, see the DSHS Statement on Johnson & Johnson Vaccine here.
As of February 27, 2021, the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for the third COVID-19 vaccine.
Johnson & Johnson’s Janssen COVID-19 Vaccine has properly met the required FDA criteria and will be provided in the United States for individuals 18 years and older. While the Pfizer-BioNTech and Moderna vaccines are still to be distributed, J&J plans to deliver over 20 million doses in March and 100 million doses within the first half of 2021.
What’s So Different About J&J’s Vaccine?
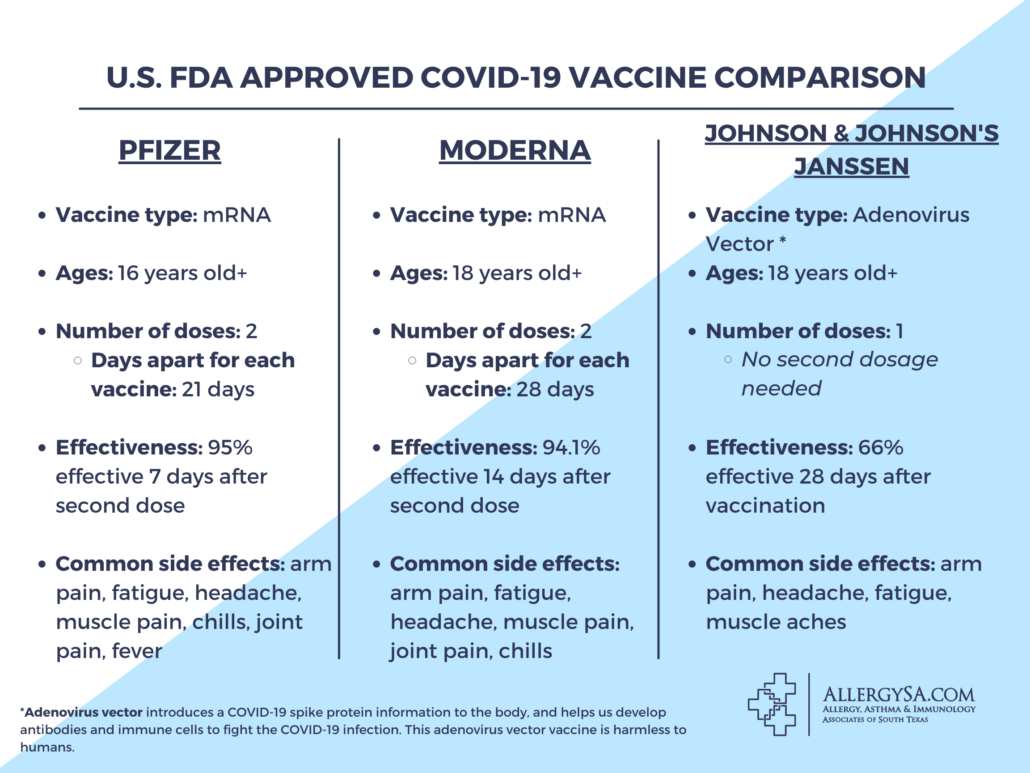
Pfizer and Moderna vaccines both have similar studies, effectiveness rates, vaccine types, etc. to prevent COVID-19 and have proven effectiveness. Johnson & Johnson’s Janssen vaccine shows various differences that are still effective and will prevent contracting COVID-19.
While Pfizer and Moderna are both mRNA (messenger RNA) vaccines, Johnson & Johnson developed a harmless adenovirus vector vaccine. This means the vaccine uses a harmless adenovirus to introduce COVID-19 spike protein information to the body, and helps us develop antibodies and immune cells to fight COVID-19 infection. Johnson & Johnson used this approach with their Ebola vaccine in July of 2020, which authorized by the European Medicines Agency.
The Janssen COVID-19 vaccine is a single-shot vaccine as opposed to the two-shot strategy that Pfizer and Moderna have developed. It is 66% effective in preventing the disease and 85% effective in preventing severe disease. These percentages are shown to be effective 28 days after vaccination. This vaccine is a useful tool in fighting the pandemic and combating the continuous high number of cases.
What Should I Do After Receiving the COVID-19 Vaccine?
Continue to wear your mask, practice social distancing, wash your hands frequently and all other practical guidelines that the CDC has provided us. As of right now, the vaccine is not quite yet out for the general public, so there are many without the vaccine that are still at risk of acquiring COVID-19.
Please continue to be cautious around those at high risk and the elderly.
It is recommended that once an individual is vaccinated, they are to be monitored 30 minutes after. If you are someone with a medication, food and/or vaccine allergy, it is advised that you keep your EpiPen alongside you at all times- before, during and after your COVID-19 vaccine.
However, medication, food and/or other vaccine allergies are NOT a contraindication for the COVID-19 vaccine.
Allergy SA will keep you updated with the latest, and will let our patients now as soon as we have the vaccine available in our clinics. To stay updated, please visit our page www.allergysa.com/covid19vaccine or call us for any questions you may have at 210-616-0882.
Share this entry
-
Share on Facebook
Share on Facebook
-
Share on Twitter
Share on Twitter
-
Share on WhatsApp
Share on WhatsApp
-
Share on Pinterest
Share on Pinterest
-
Share on LinkedIn
Share on LinkedIn
-
Share on Tumblr
Share on Tumblr
-
Share on Vk
Share on Vk
-
Share on Reddit
Share on Reddit
-
Share by Mail
Share by Mail